Recall issued for birth control tablets due to a possible packaging problem
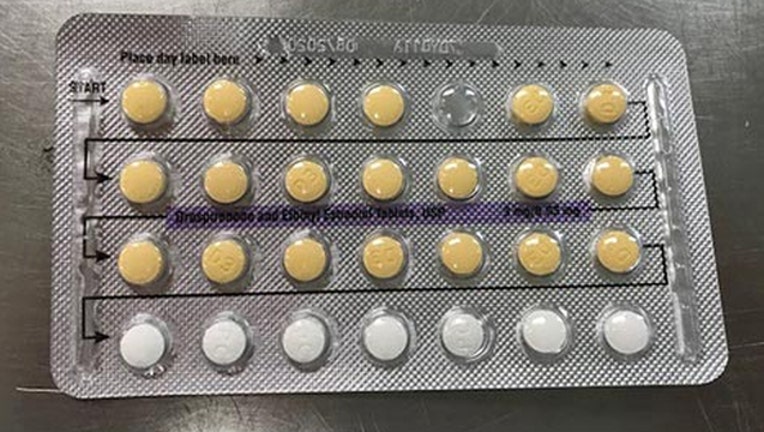
Photo shows packaging with a missing birth control tablet. On Monday, a recall was issued for birth control tablets due to a possible packaging problem (fda.gov)
(FOX 5 DC) - A voluntary recall has been issued by Apotex Corp. for birth control tablets due to a possible packaging problem, says the U.S. Food and Drug Administration.
The company is voluntarily recalling four lots of Drospirenone and Ethinyl Estradiol Tablets. They say the tablet packages may possibly contain defective blisters with incorrect tablet arrangements and/or empty blister pockets.
"As a result of this packaging error, where a patient does not take a tablet due to a missing tablet or that a patient takes a placebo instead of an active tablet, loss of efficacy is possible due to variation in the dosage consumed," read a statement released by the FDA. "To date, no case has been reported for pregnancy and adverse event to Apotex."
Patients with questions can contact Apotex corp. by phone at 1-800-706-5575 (8:30am – 5:00pm, EST Monday thru Friday) or email at UScustomerservice@Apotex.com . The FDA says patients should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
FROM FDA:
Apotex Corp. Issues Voluntary Nationwide Recall of Drospirenone and Ethinyl Estradiol Tablets, USP, 28x3 Blister Pack/Carton Due to Possibility of Missing/Incorrect Tablet Arrangement
Weston, Florida, Apotex Corp . is voluntarily recalling four lots of Drospirenone and Ethinyl Estradiol Tablets, USP to the patient /user level. The four recalled lots of Drospirenone and Ethinyl Estradiol Tablets, USP may possibly contain defective blisters with incorrect tablet arrangements and/or an empty blister pocket. The affected product is manufactured by Oman Pharmaceutical Products Co. LLC. Oman under the subcontract from Helm AG, Nordkanalstrasse 28, Hamburg, 20097, Germany.
Risk Statement : As a result of this packaging error, where a patient does not take a tablet due to a missing tablet or that a patient takes a placebo instead of an active tablet, loss of efficacy is possible due to variation in the dosage consumed. To date, no case has been reported for pregnancy and adverse event to Apotex.
Patients who have received impacted lots of Drospirenone and Ethinyl Estradiol Tablets, USP 3MG/0.03MG. or have questions regarding this recall please contact your pharmacy. Individuals should not interrupt their therapy, use a non-hormonal method of birth control, contact their health care provider for medical advice and may return the impacted packages to their pharmacist.
Drospirenone and Ethinyl Estradiol Tablets, USP are an estrogen/progestin COC indicated for use by women to prevent pregnancy. Drospirenone and ethinyl estradiol tablets (inner carton) consists of 28 film-coated, biconvex tablets in the following order: 21 yellow color tablets, each containing 3 mg drospirenone (DRSP) and 0.03 mg ethinyl estradiol (EE), and 7 placebo white color tablets. The affected Drospirenone and Ethinyl Estradiol Tablets, USP lots include the following and can be identified by NDC numbers stated on the inner and outer cartons:
- NDC number on outer carton: 60505-4183-3
- NDC Number on inner carton: 60505-4183-1
- Lot Number: 7DY008A, 7DY009A, 7DY010A, 7DY011A
- Expiration Date: 8/2020
- Strength: 3MG / 0.03MG
- Configuration/Count: Outer Carton: Contains three inner Cartons | Inner Carton: Contains 1 blister with 21 active yellow color tablets and 7 placebo white color tablets.
The affected Drospirenone and Ethinyl Estradiol Tablets were distributed Nationwide to wholesalers and distributors.
Apotex Corp. has notified its affected wholesalers and distributors via mail (FedEx Standard Overnight) by mailing a recall notification letter and is arranging for return of all recalled product. Patients / users that have the affected lots of Drospirenone and Ethinyl Estradiol Tablets, USP which are being recalled should consult with their healthcare provider.
Wholesalers, Distributors and Retailers return the impacted product to place of purchase. Anyone with an existing inventory of the product should quarantine the recalled lots immediately. Customers who purchased the impacted product directly from Apotex can call GENCO at 1- 877-674-2082 (7:00am – 5:00pm, CST Monday thru Friday), to arrange for their return.
Consumers with questions regarding this recall can contact Apotex corp. by phone-number 1-800-706-5575 (8:30am – 5:00pm, EST Monday thru Friday) or email address UScustomerservice@Apotex.com . Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report Online : www.fda.gov/medwatch/report.htm
Regular Mail or Fax : Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.

